Work in our lab covers a broad range of topics unified by Two themes:
The first one is “study of the polyadenylation assisted RNA-degradation pathway”. Through biochemistry and genetics we investigate the mechanisms of polyadenylation and degradation of RNA and the consequences of this system on RNA stability and gene expression. Because polyadenylation and degradation of RNA are universal, we move back and forth between simple and complex organisms (bacteria, Archaea, plants and animal organelles, and human cells).
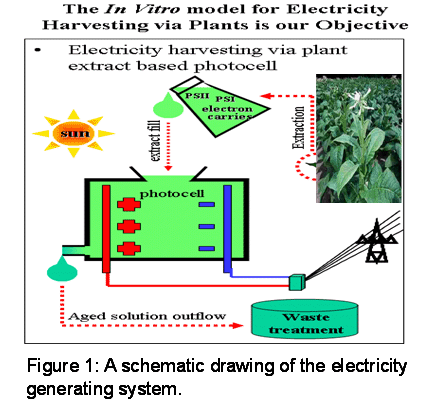
The second topic is “Harnessing photosynthesis to produce green energy in the forms of electricity and hydrogen”. We build bio-photo-electric-cells (BEPCs) where thylakoids of higher plants or photosynthetic bacteria (cyanobacteria) are used to produce electricity and hydrogen.
-
The polyadenylation stimulated RNA degradation pathway.
-
RNA degradation and polyadenylation in the chloroplast and theenzymes involved.
-
RNA degradation and polyadenylation in human mitochondria.
-
The origin of post-transcriptional addition of heteropolymeric tails to RNA in human cells.
-
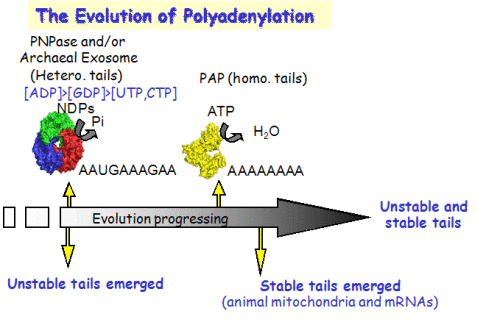
The evolution of polyadenylation; Different tails tell different tales.
-
Harnessing photosynthesis for the production of electricity and hydrogen – the Bio-photoelectric cell.
The polyadenylation stimulated RNA degradation pathway.
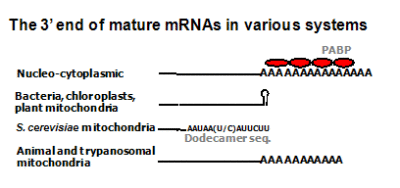
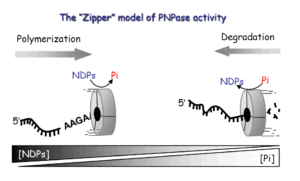
The molecular mechanism of RNA polyadenylation and degradation is one of the major focus of our research. The general scheme of this pathway consists of three sequential steps initiated with endonucleolytic cleavage of the transcript. In the second stage, the cleavage product is polyadenylated and thereby targeted for rapid exonucleolytic degradation which comprises the final step. In E. coli, polyadenylation is carried out mainly by a nucleotidyl transferase- type poly(A)-polymerase (Ntr-PAP), producing homopolymeric poly(A) tails, and to a certain extent, by PNPase, which extends heteropolymeric poly(A)-rich tails containing all four nucleotides. The exonucleolytic degradation, is performed by PNPase, RNase II, and possibly, also by RNase R. Research in E. coli led to the study of related mechanisms in other systems, revealing polyadenylation-assisted RNA degradation pathways in other bacteria, chloroplasts, both plant and human mitochondria, archaea, and for nuclear encoded transcripts in yeast and human cells. The detection of non-abundant, truncated polyadenylated RNA molecules, which are the degradation intermediates caught between the second and third stages of the pathway, serves as a tell tale sign of the presence of a poly(A)-assisted RNA degradation mechanism
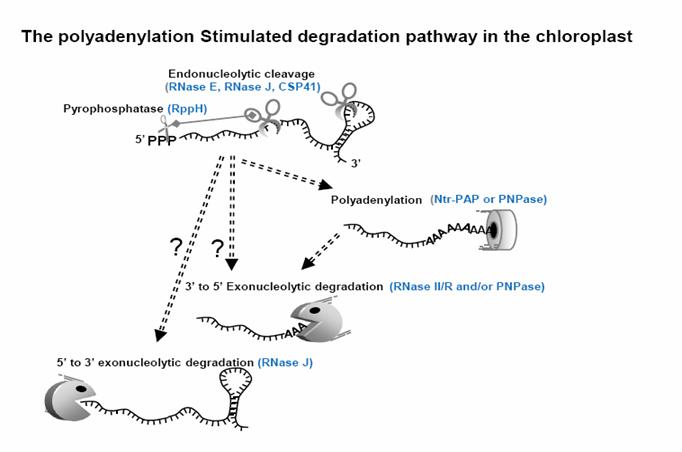
RNA degradation and polyadenylation in the chloroplast and the enzymes involved.
Gene regulation at the RNA level encompasses multiple mechanisms in prokaryotes and eukaryotes, including splicing, editing, endo- and exonucleolytic cleavage, and various phenomena related to small or interfering RNAs. Ribonucleases are key players in nearly all of these post-transcriptional mechanisms, as the catalytic agents. This research focuses into the molecular mechanism of polyadenylation and RNA degradation as well as ribonuclease activities in the chloroplast, where RNase mutation or deficiency can cause metabolic defects and is often associated with plant chlorosis, embryo or seedling lethality, and/or failure to tolerate nutrient stress. Our work has focused on the exoribonuclease polynucleotide phosphorylase (PNPase), which operates in RNA maturation and degradation. We are analyzing the structure-function of an enzyme whose importance in many cellular processes in prokaryotes and eukaryotes has only begun to be uncovered. PNPase substrates are mostly generated by endonucleolytic cleavages for which the catalytic enzymes remain poorly described. Two candidate enzymes, RNase E and RNase J are under investigation in our lab. RNase E is well-described in bacteria but its function in plants is unknown: we showed it is located in the chloroplast and catalyzes endonucleolytic cleavages. RNase J was recently discovered in bacteria but like RNase E, its function in plants has yet to be explored. The parallel in vivo and in vitro analysis allows a biologically significant correlation of biochemical and in planta results for conserved and indispensable ribonucleases. Potentially, these new insights into chloroplast gene regulation will ultimately support plant improvement for agriculture.
RNA degradation and polyadenylation in human mitochondria.
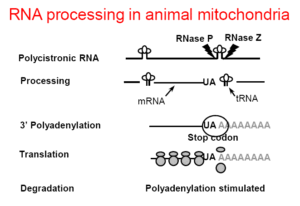
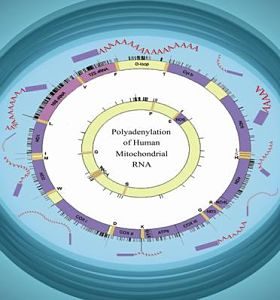
Mitochondria are DNA-containing organelles that function as the cell’s major providers of ATP and have been implicated in a variety of basic cellular processes, ranging from apoptosis to aging. Polyadenylation plays a critical role in controlling mRNA stability in many systems, including human mitochondria. Despite being key mechanisms which govern mitochondrial gene expression, RNA polyadenylation and turnover in human mitochondria, still remain unsolved puzzles. We have detected unstable, transient polyadenylation in human mitochondria, which is evidence of poly(A)-assisted RNA degradation, a process known in bacteria, chloroplasts, plants mitochondria and trypanosome mitochondria and, recently, in mammalian cell nuclei. The discovery of transient polyadenylation which occurs at sites within the mitochondrial gene sequences is especially intriguing, as it is known that stable poly(A) tails decorate the mature 3’ ends of human mitochondrial mRNA. Understanding the molecular mechanism of mRNA degradation in human mitochondria will help to understand the modulation of mRNA stability in this organelle. We also expect to obtain new and important information concerning the evolution of RNA polyadenylation, and possibly the molecular background of mitochondrial genetic disorders related to RNA degradation. Therefore, this information will not only be of great interest to those studying gene expression, but also to researchers with agendas of a medical or clinical nature.
The origin of post-transcriptional addition of heteropolymeric tails to RNA in human cells.
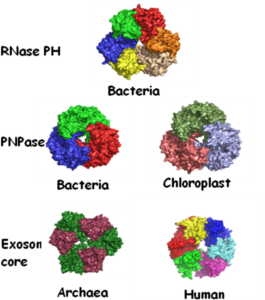
The addition of poly(A)-tails to RNA is a process common to nearly all organisms. In eukaryotes, stable poly(A)-tails important for mRNA stability and translation initiation are added to the 3’ ends of most nucleus-encoded mRNAs, but not to rRNAs and tRNAs. Contrarily, in prokaryotes and organelles, polyadenylation stimulates RNA degradation. In addition, polyadenylation of nucleus-encoded transcripts in yeast, plants, and humans was reported to promote RNA degradation, demonstrating that polyadenylation can play a double-edged role for RNA of nuclear origin. Not only homopolymeric poly(A)-tails comprised exclusively of adenosines, but also heteropolymeric poly(A)-rich extensions, including the other three nucleotides, have been observed in bacteria, archaea, chloroplasts, and human cells. These heteropolymeric poly(A)-rich tails may have a discrete function that is related to an RNA degradation mechanism or may, alternatively, represent an ancestral stage in the evolution of polyadenylation. In bacteria, archaea, and organelles, phosphorolytic activity of polynucleotide phosphorylase (PNPase) or the archaeal exosome produces heteropolymeric tails. However, the human exosome lacks phosphorolytic activity, and the identity of the enzyme that produces heteropolymeric tails in human cells is yet unknown. In this project we investigate the heteropolymeric extensions found in human cells and whether they function in promoting RNA degradation.
The evolution of polyadenylation; Different tails tell different tales.
The addition of poly(A) tails to RNA is a phenomenon common to almost all organisms. Not only homopolymeric poly(A)-tails, comprised exclusively of adenosines, but also heteropolymeric poly(A)-rich extensions, which include the other three nucleotides as well, have been observed in bacteria, archaea, chloroplasts, and human cells. Polynucleotide phosphorylase (PNPase) and the archaeal exosome, which bear strong similarities to one another, both functionally and structurally, were found to polymerize the heteropolymeric tails in bacteria, spinach chloroplasts, and archaea. As phosphorylases, these enzymes use di-phosphate nucleotides as substrates and can reversibly polymerize or degrade RNA, depending on the relative concentrations of nucleotides and inorganic phosphate. A possible scenario, illustrating the evolution of RNA polyadenylation and its related functions, is presented, in which PNPase (or the archaeal exosome) was the first polyadenylating enzyme to evolve and the heteropolymeric tails that it produced, functioned in a polyadenylation-stimulated RNA degradation pathway. Only at a later stage in evolution, did the poly(A)-polymerases that use only ATP as a substrate, hence producing homopolymeric adenosine extensions, arise. Following the appearance of homopolymeric tails, new roles for polyadenylation evolved; RNA stability and translation initiation. This was accomplished by utilizing stable poly(A) tails associated with the mature 3′ ends of transcripts. Today, stable polyadenylation coexists with unstable heteropolymeric and homopolymeric tails. Therefore, the heteropolymeric poly(A)-rich tails, observed in bacteria, organelles, archaea, and human cells, represent an ancestral stage in the evolution of polyadenylation.
Bio-photoelectric cell.
An integrated, multidisciplinary project combining plant genetic engineering, biochemistry, chemistry and electric engineering. The key objective of this multidisciplinary project is to construct a novel, sustainable energy production system that enables the production of reducing power by a crude photosynthetic extract of a transgenic plant. The system is engineered such that the electron source (water) will be replenished continually, and the only reaction by-product – molecular oxygen and hydrogen, will be removed and collected. Therefore, this bio-energy generator will produce electricity and hydrogen, both extremely valuable resources of energy. The planned system is completely “green” in function, with absolutely no production of chemical pollutants. In collaboration with Prof. Noam Adir, Faculty of Chemistry.